Every person living with neurofibromatosis type 1 (NF1) has a story—and nearly all of those stories include cutaneous neurofibromas (cNFs). These benign tumors of the skin may not pose a life-threatening risk, but for the 98% of people with NF1 who develop them, the impact can be profound: socially, emotionally, physically, and functionally.
A new clinical trial designed in collaboration between NTAP, academic, and industry partners aims to change the outlook for people living with cNF. The study is testing whether mirdametinib, a targeted therapy recently approved by the FDA for plexiform neurofibromas, can also offer relief from the debilitating burden of cutaneous tumors in adults. “We know that people with neurofibromatosis type 1 develop cutaneous neurofibromas in about 98% of cases, and despite being histologically benign tumors, they can seriously affect quality of life,” Dr. Carlos Romo, principal investigator of the study at the Johns Hopkins Hospital explained. “Neurofibromas can be painful, itchy, and disfiguring. Studies show that patients often cite them as the most bothersome manifestation of NF1. The search for treatments demands that we take that patient experience very seriously.”
Historical Collaboration Between Industry and Academia to Develop Better Treatments for cNFs
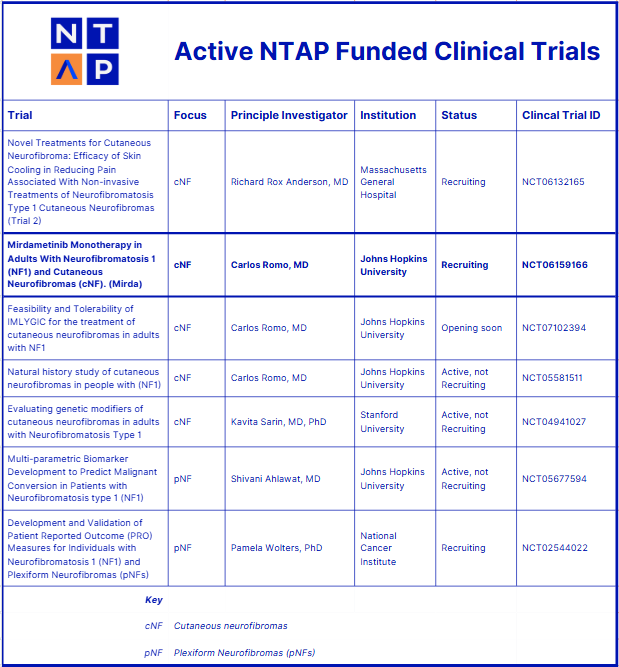
The study, titled “Mirdametinib Monotherapy in Adults With Neurofibromatosis 1 (NF1) and cNF,” launched in February 2024. It is an open-label Phase 1/2a clinical trial designed to assess the feasibility, safety, and efficacy of using mirdametinib to treat cNFs in adults with NF1. Mirdametinib, developed by SpringWorks Therapeutics, is an oral MEK inhibitor that in February 2025 received FDA approval for NF1-associated plexiform neurofibromas. This NTAP-led clinical trial with SpringWorks collaboration, through clinical trial oversight and provision of drug, is evaluating whether it may also benefit adults with cNF.
“Currently, people with cNFs are sometimes offered surgery, electrodessication, or laser treatments, but some of these treatments are not widely available; they can only target a limited number of tumors and leave scars behind. This makes treatment difficult in cases where the tumor burden is very high. By testing mirdametinib, we are hoping to find a way to treat the entire skin, potentially reaching all of the cutaneous neurofibromas,” explains Dr. Romo.
One of the study’s innovative elements is its use of 3D full-body imaging to monitor whole-skin tumor burden. The Vectra 360 system, originally developed by Canfield for dermatology and plastic surgery, allows researchers to capture and analyze nearly the entire skin surface in high resolution, a significant improvement on manual methods alone. It’s an approach that has not been applied for interventional NF1 clinical trials before and offers a more complete picture of skin tumor burden and how it changes over time.
Exploring the Relationship Between Clinician Reported Outcomes and Patient Reported Outcomes
The trial design front-loads a high frequency of in-person evaluations at Johns Hopkins—approximately nine clinic visits in the first three months—so the team can measure cNFs with digital calipers, capture full-body 3D images of nearly the entire skin surface, and obtain blood and cNF tissue samples. At the same visits, participants complete patient-reported outcome questionnaires about pain, appearance, and quality of life. This delivers validated clinician and patient-reported outcomes, allowing the study to directly compare clinical outcomes with the patient’s own experience.
“If a tumor doesn’t shrink, but stops itching, or blends in better with surrounding skin, that can still be a meaningful win for the patient,” Dr. Romo added. “That’s at least as high a level of success as saying it decreased by five millimeters.”
A Clinical Trial Designed Collaboratively, Conducted Independently
The trial is co-designed and co-chaired by Dr. Jaishri Blakeley of NTAP, a neuro-oncologist at Johns Hopkins University, Dr. Lu Le of the University of Virginia, and Dr. Pierre Wolkenstein of Hôpital Henri-Mondor and Université Paris-Est Créteil—two of the world’s foremost dermatology experts in NF1 research. NTAP played a central role, not only in funding the work, but in bringing these investigators together with the pharmaceutical partner SpringWorks Therapeutics. By coordinating across institutions and continents, NTAP created the structure and momentum needed to launch the study, ensuring the right people, resources, and focus were in place.
Along with funding, NTAP’s stewardship ensures scientific independence; while SpringWorks Therapeutics provides the study drug, it is not involved in data collection, analysis, or interpretation. It’s the ideal partnership between industry, investigators, and patients to drive innovation and de-risk investment in an underserved area.
Looking Ahead
This study is exploring the possibility of a systemic treatment for adults with cNF, holding out the promise of improving quality of life for people with NF1 by treating one of the most common and previously understudied manifestations of NF1.
“There is a tendency in the field to pay less attention to cNFs because these are benign tumors. However, we know that they are a big problem for many patients with NF1, and we hope that a systemic therapy might represent an important improvement over destructive local therapies like the ones that are mostly available now.”
As of September 2025, 8 out of 18 participants are enrolled in the trial, with some nearing the end of their two-year course of treatment. While it’s still too early to draw formal conclusions, retention has been high, which is an encouraging sign. “At this stage, we cannot make any claims about the results, but the retention rate of our study patients has been excellent,” explains Dr. Romo. “Despite an intense schedule of visits, measuring and reporting, participants remain committed to this project. I am hoping that this is a sign of them finding some benefit.”
For more information on the Mirdametinib Monotherapy in Adults With Neurofibromatosis 1 (NF1) and cNF clinical trial please visit ClinicalTrials.Gov.
